Nrp Unique Challenges of a Premature Baby During Resuscitation
Abstruse
Background
Nitric oxide (NO) plays an important role in normal postnatal transition. Our aims were to determine whether calculation inhaled NO (iNO) decreases supplemental oxygen exposure in preterm infants requiring positive pressure ventilation (PPV) during resuscitation and to study iNO furnishings on middle rate (HR), oxygen saturation (SpOii), and demand for intubation during the showtime 20 min of life.
Methods
This was a pilot, double-blind, randomized, placebo-controlled trial. Infants 25 0/7–31 vi/7 weeks' gestational age requiring PPV with supplemental oxygen during resuscitation were enrolled. PPV was initiated with either oxygen (FiO2–0.30) + iNO at 20 ppm (iNO group) or oxygen (FiOtwo–0.xxx) + nitrogen (placebo group). Oxygen was titrated targeting defined SpO2 per electric current guidelines. After ten min, iNO/nitrogen was weaned stepwise per protocol and terminated at 17 min.
Results
20-eight infants were studied (14 per group). The hateful gestational historic period in both groups was similar. Cumulative FiO2 and charge per unit of exposure to high FiO2 (>0.lx) were significantly lower in the iNO grouping. There were no differences in HR, SpOii, and need for intubation.
Conclusions
Assistants of iNO every bit an adjunct during neonatal resuscitation is feasible without side effects. It diminishes exposure to high levels of supplemental oxygen.
Introduction
Survival of extremely premature babies has improved in the past 2 decades.ane,2,3 Still, morbidities associated with prematurity, such as necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), patent ductus arteriosus (PDA), neurodevelopmental delay, bronchopulmonary dysplasia (BPD), and retinopathy of prematurity (ROP) present a pregnant burden for surviving premature infants.1,ii,three Of these comorbidities, the latter two are associated with oxygen use and mechanical ventilation.4,5,6,7 At present, optimal inspired oxygen concentration for premature infants during resuscitation is nonetheless uncertain. Electric current resuscitation guidelines recommend maintaining set oxygen targets past minimizing oxygen exposure subsequently birth.8,9 Assistants of high levels of oxygen during resuscitation to maintain target oxygen saturation may be harmful, due to the generation of reactive oxygen species.10
Transition from fetal to neonatal apportionment involves a complex sequence of events during which pulmonary vascular resistance drops dramatically in association with an increment in peripheral vascular resistance.xi,12,13,xiv This cardiovascular accommodation at birth is facilitated by the release of cortisol, catecholamines, and vasodilator mediators, such as nitric oxide (NO) and prostaglandin (PG) I2.15,16,17,18,19 NO is particularly important equally it regulates pulmonary vascular tone at birth.xx,21
NO is released from the vascular endothelium and plays a critical role in vascular adaptation during the perinatal menses.22,23 It is a powerful vasodilator, regulates pulmonary vascular tone, facilitates fluid clearance from the alveoli, and secures the integrity of the endothelial barrier.22,23 It activates vascular smooth muscle cell soluble guanylate cyclase and cyclic guanosine monophosphate production, leading to pulmonary smooth musculus relaxation, through the regulation of intracellular calcium levels.23,24 The major accent of current resuscitation guidelines is on ventilation, with the expectation that pulmonary vasodilation will follow.viii,9 Indeed, ventilation of the lungs with air alone promotes reduction in pulmonary vascular resistance by release of vasoactive mediators.xiv However, these guidelines practice not provide any boosted steps to facilitate pulmonary vasodilation other than oxygen administration and lung expansion.
Both animal and human studies have shown that inhaled NO (iNO) decreases pulmonary vascular resistance during hypoxemia.25,26 Therefore, in premature infants needing resuscitation, adding iNO during resuscitation may subtract pulmonary vascular resistance, improve ventilation perfusion mismatch, and reduce the need for excess oxygen. In hypoxic near-term lamb model, exposure to twenty ppm of iNO at birth decreased pulmonary vascular resistance and resulted in an increase in pulmonary blood period.22 iNO assistants during resuscitation has never been attempted in human newborns.
The objective of this written report was to test the hypothesis that iNO, added equally an offshoot to positive pressure ventilation (PPV), may reduce the need for supplemental oxygen and may advance the transition to neonatal circulation during resuscitation in premature infants.
Our primary aims were: (ane) to determine whether adding iNO decreases supplemental oxygen exposure in preterm infants requiring PPV during resuscitation as per NRP guidelines and (ii) to compare centre rate (Hour), oxygen saturation, and demand for intubation between the two groups during the start twenty min of life.
Our secondary aims were: (one) to evaluate the consequence of iNO during resuscitation on immediate postnatal respiratory support in the first 24 h and (2) to evaluate the effect of iNO on the incidence of IVH, NEC, tardily-onset sepsis, PDA requiring treatment, ROP, BPD, mortality, and Neonatal Intensive Care Unit length of stay.
Methods
Study design and patients
This double-blind, randomized, placebo-controlled, pilot trial was approved by the Academy of Oklahoma Health Sciences Center Institutional Review Board. Written informed consent was obtained prior to commitment. The written report was registered with NIH Clinical Trials (Clinicaltrials.gov, identifier NCT01220687). New investigational drug approval was obtained from the Nutrient and Drug Administration (IND110317).
The target sample size for this pilot study was 40 (20 newborns in each arm). The capricious sample size of 20 per group was chosen as it was accounted large plenty to fairly cover the whole spectrum of the studied gestational age. Inclusion criteria were newborn 25 0/7–31 6/7 weeks' gestational historic period requiring continuous positive airway pressure or PPV during commitment room (DR) resuscitation/stabilization. Exclusion criteria were known complex congenital anomalies of the center or lungs and hydrops fetalis.
Randomization and masking
Infants meeting these criteria were randomly assigned to receive either oxygen plus iNO diluted in Due north2 carrier gas (iNO grouping) or oxygen in N2 carrier gas alone (placebo group). Subjects were randomized using blocks of size two or four, chosen randomly, to ensure proper balance of patients in each handling arm throughout the study. To ensure proper blinding, and because of the newborn's need for resuscitation, and thus report inclusion was unknown a priori, equipment set-up based on the randomization group was performed in advance. This was done so that DR personal would be unaware of the treatment infants received, if resuscitation was administered.
Procedures
All neonatal resuscitations were done following NRP guidelines (sixth edition).27 Immediately subsequently nascence, monitoring sensors were placed for pre- (correct arm) and postal service- (feet) ductal saturations using two pulse oximeters (Masimo Set Radical-7 RDS-3™, Irvine, CA, The states). Betwixt thirty and 90 south, if the infant required PPV, those assigned to the iNO group received 30% oxygen and the study gas. A dose of xx ppm iNO is the standard approved dose for neonates with persistent pulmonary hypertension of newborn (PPHN) and has been shown to be rubber in preterm infants during early postnatal periods.28 Those in the placebo group received 30% oxygen and nitrogen added to the oxygen source via a delivery port built into the excursion. At x min of respiratory support, the study gas (iNO or placebo) was downward-titrated by 5 ppm per minute until the concentration reached five ppm, then reduced every minute by one ppm until study gas was terminated (a full of 17 min). Data collection continued for another 3 min and ended at 20 min (Fig. 1).
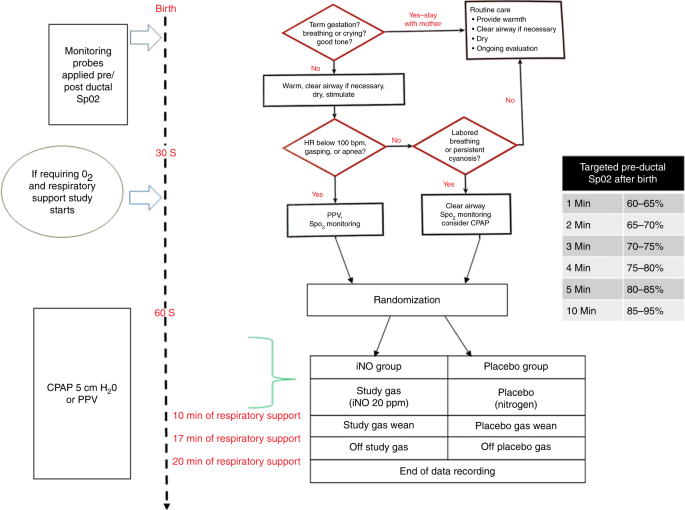
NRP algorithm (6th edition) and study procedures
A T-piece resuscitator (NeoPuff™, Fisher & Paykel Healthcare, Auckland, New Zealand) was used for PPV during initial respiratory support. Oxygen assistants was titrated to go along pre-ductal saturations within the target range (ane min sixty–65%, 2 min 65–lxx%, 3 min 70–75%, 4 min 75–fourscore%, 5 min 80–85%, and 10 min 85–95%). Positive-end expiratory pressure was kept constant at five cm of water. Peak inspiratory pressure was adapted to ensure optimal breast rise. No surfactant was administered during the first 20 min later on initiation of PPV.
The information capture device for this written report was congenital effectually the iNO vent, as follows:
The respiratory support department consisted of a blender, T-piece resuscitator, and warmer/humidifier with an injector module to deliver study gas (iNO) or nitrogen (placebo) with positive pressure level treatment.
The patient monitoring system consisted of pre- and post-ductal saturations, Hour, in-line FiOtwo, and pressure monitor for the T-piece resuscitator.
The INO delivery/monitor consisted of an INOVENT Delivery Organization™ (Model number 1605-9000-000, Datex Ohmeda, Mallinckrodt, USA), which includes an in-line oxygen analyzer, NO, NOtwo, and delivered oxygen analyzer.
The whole set-up occupied the space of a standard ship incubator.
Data capture and drove
Data were continuously nerveless during the study via an integrated data acquisition system and software (BioPac™ MP 150 Data Acquisition Arrangement, BIOPAC Systems, Inc. USA). Real-time brandish consisted of a report clock, pre-ductal saturation, Hr, analyzed and delivered FiO2, and PPV pressure waveform. The resuscitation team was blinded to both iNO delivery and display on the front of the INO vent. Prior to the study, bench testing of this device was performed in a SimnewB® non-breathing model to ensure accurate delivery of oxygen and iNO to the nasal and hypo-pharyngeal surface area with the T-Slice resuscitator. This test verified that in that location was no difference betwixt the analyzed and the delivered oxygen.
Statistical analysis
Descriptive statistics were computed for all demographic and clinical variables. For bivariate group comparisons of continuous variables, data were commencement assessed for normality using Shapiro–Wilk test then compared using Student's t test or Wilcoxon–Isle of mann–Whitney examination, equally appropriate. Similarly, bivariate group comparisons of categorical variables used the chi-square or Fisher's verbal test as appropriate. The biometric data captured were and so sampled at ane observation every v southward, for a total of 240 observations per patient, for clarity and assay. Max FiO2, cumulative FiOtwo exposure, FiOii at study terminate, HR, and pre- and mail service-ductal saturation percentages were calculated from these measures for both groups, assessed for normality, and compared using Pupil'due south t exam or Wilcoxon–Mann–Whitney test, as appropriate. The charge per unit of hyperoxia (FiOtwo > 0.60) over the written report period was compared betwixt the two groups using Poisson exam. In addition, functional master component (PC) analysis was used to estimate hateful functional curves of the biometrics over the study flow and to place contiguous time points of significant variability between groups for each outcome. PC scores were assigned to each subject field inside regions of high variation and the scores between groups were compared using Anderson–Darling exam.29,30
Results
This study was conducted between March, 2012 and February, 2017. Of the 171 mothers eligible for study participation, 95 provided written informed consent and were included. Of these 95 candidates, 61 were excluded. Thirty-four infants were randomized at birth. Enrollment procedure was discontinued prior to reaching our target (n = 40) due to deadening recruitment. In all, 28 infants (14 in each group) were included in last analysis (Fig. 2). No significant inter-grouping differences were institute in patient demographics (Tabular array one).
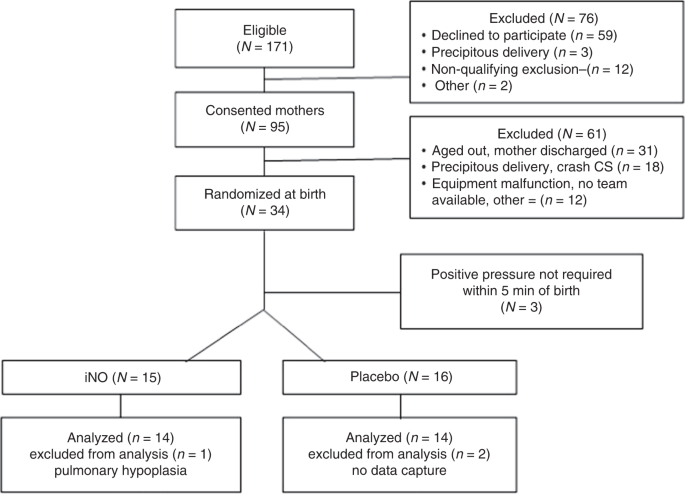
Study enrollment and randomization
Oxygen during resuscitation
Infants in the placebo group responded to PPV with oxygen (+nitrogen) only required higher oxygen throughout resuscitation compared to the iNO group. Infants randomly assigned to the iNO (+oxygen) group received significantly less cumulative FiOtwo when summed across all sample points in the study period (p = 0.0012). In addition, the maximum inspired oxygen required during the study in the iNO group tended to be lower every bit compared to the placebo group (0.39 vs. 0.48; p = 0.0536). This trend became more than pronounced at the finish of 20 min betwixt the iNO and the placebo group, the iNO group still receiving lower oxygen concentration compared to the placebo group (26% vs. 36%; p = 0.0326; Fig. 3). The placebo group had 9 hyperoxia (FiO2 > 0.60) subjects combining for 708 FiO2 measures >0.60 over the study period compared to 6 subjects in the iNO grouping combining for 370 FiO2 measures >0.60 over the study menstruation. The rate ratio of hyperoxia for the placebo group compared to the iNO grouping was one.91 (95% conviction interval = 1.68, 2.xviii; p < 0.0001) indicating that the rate of oxygen exposure >0.lx was significantly higher in the placebo group than in the iNO group (Fig. 3).
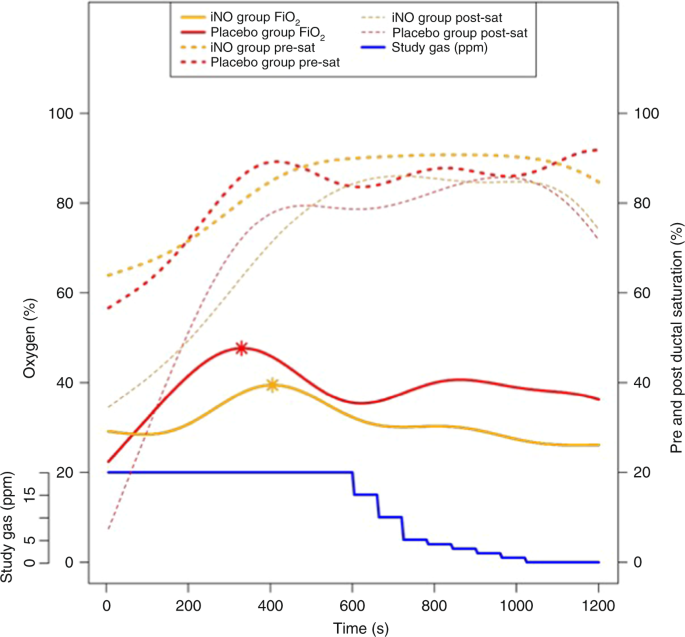
Oxygen delivery and pre- and postal service-ductal oxygen saturations during resuscitation. The solid lines (xanthous and red) correspond average oxygen administered to each group. The interrupted lines (yellow and cherry) correspond pre-ductal (thick) and post-ductal (thin) oxygen saturations in each group. The asterisks stand for the highest average oxygen concentration in each group. The blue line represents written report gas (ppm)
60 minutes during resuscitation
Throughout the resuscitations, the average HR appeared to be lower in the iNO grouping than in the placebo group (Fig. 4). Mean HR for iNO vs. placebo was 130 ± 46 vs. 139 ± 53 beats per minute (bpm) at 5 min (p = 0.920) and 149 ± 46 vs. 161 ± 53 bpm at 10 min (p = 0.354). At the end of 20 min, hateful HR was 153 ± 46 vs. 168 ± 53 bpm. However, these differences were not statistically meaning (p = 0.111) (Fig. 4).
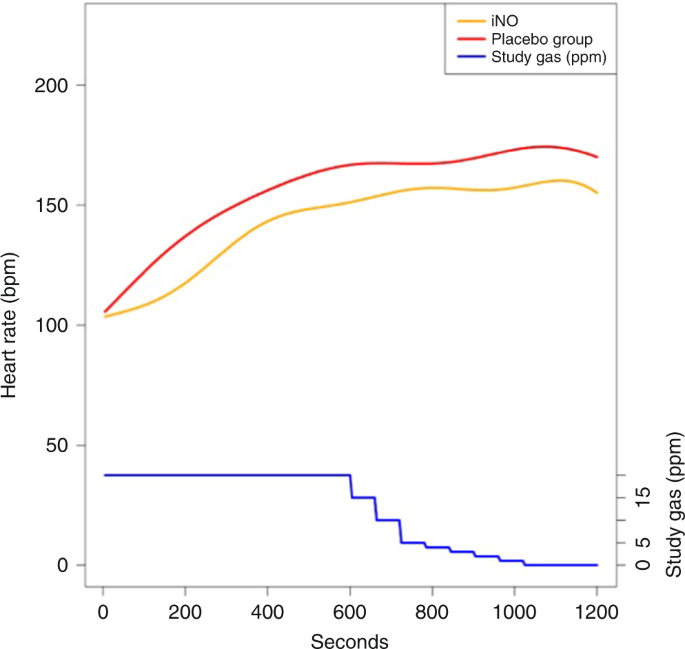
Heart charge per unit during resuscitation. The yellow and red lines stand for average heart charge per unit for the iNO and placebo groups, respectively. The blueish line represents study gas (ppm)
Pre- and post-ductal saturation
Mean pre- and mail service-ductal saturations (%) were not significantly unlike between the groups at the end of the study (Pre: placebo 90.v ± v.7, iNO 82.8 ± 13.8; p = 0.757 and Mail: placebo 79.nine ± 25.viii, iNO 78.2 ± twenty.0; p = 0.849) (Fig. iii).
Demand for intubation
Twenty-nine percent of babies in each group were intubated during resuscitation (iNO iv/14, Placebo 4/14; p = i.0).
Secondary outcomes
At that place were no meaning group differences in whatsoever secondary outcomes (Table 2). At that place were no betwixt-grouping differences in the need for supplemental oxygen or ventilator back up in the 24 h post-obit birth (data not shown).
There were no adverse events noted during resuscitation.
Word
This preliminary report demonstrated that administration of iNO as an offshoot during neonatal resuscitation is viable without side effects. Providing iNO in addition to oxygen during PPV for extremely premature infants at nascency is associated with a decreased need for supplemental oxygen treatments. Although the iNO group appeared to have a lower average HR, it was not statistically different.
This report was designed to determine whether the add-on of iNO would reduce the oxygen needed when premature neonates require PPV, with or without intubation. Elevated pulmonary artery pressure in the fetus, or "physiologic pulmonary hypertension," may persist in the immediate postnatal period and may not respond to ventilation alone, as NRP guidelines recommend. In these situations, iNO may augment pulmonary vasodilation, which is essential in the prenatal-to-perinatal pulmonary transition, just as it does in infants with PPHN.31
Studies of iNO in preterm infants with hypoxemia have shown mixed results. In fauna studies, iNO showed anti-inflammatory backdrop, stimulated angiogenesis, increased alveolar growth, improved surfactant function, and inhibited proliferation of polish muscle cells.32 In large randomized trials in premature infants, none of the benefits seen in animal models were seen when iNO was administered to reduce inflammation and chronic lung disease,33 although a subset of preterm infants appeared to do good from it.28,34 Notwithstanding, these trials have demonstrated that iNO is safe and does not increase oxidative stress or lung inflammation. iNO has never been evaluated in newborn resuscitation for its vasodilator properties, which is essential for extra-uterine accommodation.
In neonatal lambs resuscitated with 21%, 50%, or 100% oxygen ventilation, those with 50% and 100% oxygen experienced a greater initial subtract in pulmonary vascular resistance compared with those resuscitated with 21% with blunted subsequent response to iNO subsequently in the 100% oxygen group. Asphyxiated lambs resuscitated with 100% oxygen showed increased superoxide anions in pulmonary arteries compared to 21% oxygen resuscitation.26,35 These observations advise that resuscitation with high oxygen concentrations should be avoided in premature infants equally information technology may produce morbidities, such as ROP and BPD.i,2,3 This has been shown in premature infants where resuscitation with 30% compared to 90% oxygen causes less oxidative stress and bronchopulmonary dysplasia.10 Therefore, less oxygen exposure in premature infants during resuscitation is preferable, and iNO during resuscitation may produce these desired benefits. Every bit observed in this study, iNO group required less supplemental oxygen over the entire written report period. In addition, in that location was less exposure to hyperoxia (FiO2 > 0.lx). This is probably due to the vasodilator properties of iNO, improving ventilation–perfusion mismatch, and reducing the need for supplemental oxygen to maintain saturation targets. It is interesting to note that the iNO group maintained a lower oxygen requirement starting at 5–half dozen min of resuscitation, even as the iNO was slowly downwardly-titrated, at x min of resuscitation. This finding indirectly indicates that the immediate vasodilator event of iNO appears to be sustained, despite having a very brusk half-life (3–five s). In contrast, the placebo grouping, after an initial positive response to ventilation, began requiring greater supplemental oxygen after 10 min.
The trend toward a lower HR in the iNO group was an interesting observation. Although non statistically meaning, at 5, 10, and 20 min the boilerplate Hour was 9, 12, and 15 bpm lower respectively in this group. 60 minutes is the main determinant of cardiac output in newborns. Cardiac output is dependent on the HR, contractility, preload, and afterload.36 We speculate that the observed differences may exist due to improved lung perfusion associated with improved oxygenation and meliorate venous render to the left side of the heart and increased left ventricular output. Therefore, improved pulmonary claret menstruation (i.e., enhanced preload) may be responsible for maintaining cardiac output and keeping pre-ductal saturation within the targeted range in the iNO grouping despite a lower Hour. In improver, the mean Hr in this study was similar to resuscitation studies using ninety% and 100% oxygen in premature babies.37
The secondary outcomes were not unlike between the groups. In item, the absence of IVH > 2 in either grouping is consistent with previous observations in big clinical trials in premature infants.28,33
One of the limitations of our study is the small sample size, but this was known prior to study initiation and is consistent with its pattern as a pilot project. We stopped recruitment before reaching our initial target sample size. The study design required consent prior to delivery, which required screening, and consenting from a large number of parents. In addition, several consented mothers were not enrolled due to abrupt delivery of extremely premature babies, unavailability of the team, or the infant aged out for enrollment. Nevertheless, we believe our concluding sample size was acceptable as a airplane pilot trial.
Conclusions
Administration of iNO as an adjunct gas during neonatal resuscitation is feasible and decreases the amount of exposure to supplemental oxygen in very preterm infants. This was not associated with any adverse events. These findings suggest that iNO facilitates cardiopulmonary transition at birth. This novel approach should be validated by large randomized trials.
References
-
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
-
Horbar, J. D. et al. Bloodshed and neonatal morbidity amongst infants 501 to 1500 grams from 2000 to 2009. Pediatrics 129, 1019–1026 (2012).
-
Shah, P. S. et al. Outcomes of preterm infants <29 weeks gestation over 10-twelvemonth period in Canada: a cause for concern? J. Perinatol. 32, 132–138 (2012).
-
Jobe, A. H. & Kallapur, S. G. Long term consequences of oxygen therapy in the neonatal flow. Semin. Fetal Neonatal Med. 15, 230–235 (2010).
-
Birenbaum, H. J. et al. Chronic lung disease in very low nascence weight infants: persistence and improvement of a quality improvement procedure in a tertiary level neonatal intensive intendance unit. J. Neonatal Perinat. Med. nine, 187–194 (2016).
-
Finer, N. N. et al. Early CPAP versus surfactant in extremely preterm infants. N. Engl. J. Med. 362, 1970–1979 (2010).
-
Carlo, Westward. A. et al. Target ranges of oxygen saturation in extremely preterm infants. N. Engl. J. Med. 362, 1959–1969 (2010).
-
Wyllie, J. et al. Part seven: Neonatal resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Handling Recommendations. Resuscitation 95, e169–e201 (2015).
-
Weiner, Thousand. Textbook of Neonatal Resuscitation (American Academy of Pediatrics, 2016).
-
Vento, M. et al. Preterm resuscitation with depression oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 124, e439–e449 (2009).
-
Lakshminrusimha, S. & Steinhorn, R. H. Pulmonary vascular biology during neonatal transition. Clin. Perinatol. 26, 601–619 (1999).
-
Gao, Y. & Raj, J. U. Regulation of the pulmonary apportionment in the fetus and newborn. Physiol. Rev. 90, 1291–1335 (2010).
-
Hillman, Northward. H., Kallapur, Southward. Grand. & Jobe, A. H. Physiology of transition from intrauterine to extrauterine life. Clin. Perinatol. 39, 769–783 (2012).
-
Teitel, D. F., Iwamoto, H. Due south. & Rudolph, A. M. Changes in the pulmonary circulation during birth-related events. Pediatr. Res. 27, 372–378 (1990).
-
Behrman, R. Eastward. & Lees, 1000. H. Organ blood flows of the fetal, newborn and developed rhesus monkey: a comparative written report. Biol. Neonate 18, 330–340 (1971).
-
Heymann, G. A., Lewis, A. B. & Rudolph, A. M. Pulmonary vascular responses during advancing gestation in fetal lambs in utero. Chest 71, 270–271 (1977).
-
Lewis, A. B., Heymann, M. A. & Rudolph, A. M. Gestational changes in pulmonary vascular responses in fetal lambs in utero. Circ. Res. 39, 536–541 (1976).
-
Lakshminrusimha, S. et al. Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen. J. Appl. Physiol. (1985) 111, 1441–1447 (2011).
-
Lakshminrusimha, S. The pulmonary circulation in neonatal respiratory failure. Clin. Perinatol. 39, 655–683 (2012).
-
Abman, South. H., Chatfield, B. A., Hall, Southward. L. & McMurtry, I. F. Part of endothelium-derived relaxing gene during transition of pulmonary apportionment at birth. Am. J. Physiol. 259, H1921–H1927 (1990).
-
Fineman, J. R., Wong, J., Morin, F. C. 3rd, Wild, L. K. & Soifer, S. J. Chronic nitric oxide inhibition in utero produces persistent pulmonary hypertension in newborn lambs. J. Clin. Invest. 93, 2675–2683 (1994).
-
Roberts, J. D. Jr. et al. Inhaled nitric oxide reverses pulmonary vasoconstriction in the hypoxic and acidotic newborn lamb. Circ. Res. 72, 246–254 (1993).
-
Cassin, S. The office of eicosanoids and endothelium-dependent factors in regulation of the fetal pulmonary circulation. J. Lipid Mediat 6, 477–485 (1993).
-
Lakshminrusimha, S. & Saugstad, O. D. The fetal circulation, pathophysiology of hypoxemic respiratory failure and pulmonary hypertension in neonates, and the office of oxygen therapy. J. Perinatol. 36(Suppl ii), S3–S11 (2016).
-
Kinsella, J. P. & Abman, S. H. Clinical approach to inhaled nitric oxide therapy in the newborn with hypoxemia. J. Pediatr. 136, 717–726 (2000).
-
Lakshminrusimha, S. et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr. Res. 66, 539–544 (2009).
-
Kattwinkel, J. Textbook of Neonatal Resuscitation (American Academy of Peditarics/American Middle Association, Chicago, 2011).
-
Schreiber, M. D. et al. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. Northward. Engl. J. Med. 349, 2099–2107 (2003).
-
Lin, Due north., Jiang, J., Guo, S. & Xiong, M. Functional principal component analysis and randomized sparse clustering algorithm for medical prototype analysis. PLoS Ane ten, e0132945 (2015).
-
Greven, S., Crainiceanu, C., Caffo, B. & Reich, D. Longitudinal functional principal component analysis. Electron. J. Stat. iv, 1022–1054 (2010).
-
Lakshminrusimha, S., Konduri, G. G. & Steinhorn, R. H. Considerations in the direction of hypoxemic respiratory failure and persistent pulmonary hypertension in term and tardily preterm neonates. J. Perinatol. 36, S12–S19 (2016).
-
McCurnin, D. C. et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin degradation in a baboon model of neonatal chronic lung illness. Am. J. Physiol. Lung Prison cell. Mol. Physiol. 288, L450–L459 (2005).
-
Barrington, K. J., Finer, North., Pennaforte, T. & Altit, Chiliad. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst. Rev. CD000399 (2017).
-
Ballard, R. A. et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N. Engl. J. Med. 355, 343–353 (2006).
-
Lakshminrusimha, S. et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr. Res. 62, 313–318 (2007).
-
Vincent, J. L. Understanding cardiac output. Crit. Care 12, 174 (2008).
-
Oei, J. L. et al. Targeted oxygen in the resuscitation of preterm infants, a randomized clinical trial. Pediatrics 139, e20161452 (2017).
Acknowledgements
We give thanks Karen Corff, Clara Song, and Ted Jeffers for obtaining consent from the parents; Susan James, Stephanie Montgomery, and Gary Revas for randomization and equipment set-up in the delivery room; Richard Wade, UCSD, USA for providing technical back up for the study; Doug Swanton, Mallinckrodt Pharmaceuticals, USA for back up during demote testing; and Factor Halford for editing and reviewing the manuscript. We besides thank the members of the Data Prophylactic Monitoring Board: R. Ramanathan, MD, and P. Friedlich, Md, MS Epi, MBA, University of Southern California, U.s.a.. This study was funded by an unrestricted grant from Mallinckrodt Pharmaceuticals, USA.
Author information
Affiliations
Contributions
K.S. and South.N. conceptualized and designed the study. Thou.S. wrote the commencement draft of the manuscript. E.South., Due south.Northward., and Southward.Fifty. reviewed and revised the manuscript. M.A. analyzed the data and provided the statistical support. M.Thou. congenital the iNO commitment and monitoring system and nerveless the data. M.M., A.W., D.D., A.H., J.R., and A.Yard. obtained informed consent and conducted the study in the DR. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sekar, K., Szyld, Due east., McCoy, M. et al. Inhaled nitric oxide as an offshoot to neonatal resuscitation in premature infants: a pilot, double blind, randomized controlled trial. Pediatr Res 87, 523–528 (2020). https://doi.org/10.1038/s41390-019-0643-x
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
Result Date:
-
DOI : https://doi.org/10.1038/s41390-019-0643-x
Further reading
Source: https://www.nature.com/articles/s41390-019-0643-x?error=cookies_not_supported&code=6b12b871-ea9a-4c00-8fb3-de05fe1f3e93
Post a Comment for "Nrp Unique Challenges of a Premature Baby During Resuscitation"